Refine by
Implant Materials Equipment Supplied In Usa
32 equipment items found
Manufactured by:Cook Biotech Inc. based inWest Lafayette, INDIANA (USA)
The Biodesign Otologic Repair Graft is intended for use as an implant material to aid in surgical repairs and as an adjunct to aid in the natural healing process in various otologic procedures, including but not limited to myringoplasty and ...
Manufactured by:U.S. Titanium Industry Inc. (USTi) based inBaldwin Park, CALIFORNIA (USA)
Medical titanium plate, sheet, bar, rod, wire and forging titanium orthopedic implants. Material: GR23, Ti-6AL-4V ASTM F136 ISO5832-3. GR2, GR3, GR4, ASTM F67 ...
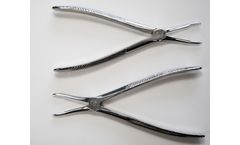
Manufactured by:GraMedica based inMacomb, MICHIGAN (USA)
These Foot Implant Forceps provides surgeons with the ability to grasp and extricate any titanium extra-osseous stent. These forceps minimize tissue handling and can assist in the removal of other implanted materials such as k-wires, screws or pins. ...
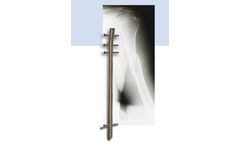
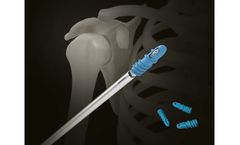
Manufactured by:Ortho-Medical GmbH based inDuerbheim, GERMANY
Implants for locking humeral nailing. System of implants: Material: titanium alloy Locking humeral nail, cannulated, diameter 7 - 8 - 9 mm. Locking screw, diameter 3,9 mm, End cup, Surgical set for locking humeral ...
Manufactured by:UroLift System based inPleasanton, CALIFORNIA (USA)
UroLift Implant: the obstructing prostatic lobes are held apart by small permanent UroLift Implants that are deployed by the delivery device. Each implant is made with common implantable materials: nitinol, stainless steel, and PET suture. Typically, four implants are placed into the ...
Manufactured by:Peters Surgical based inBoulogne-Billancourt, FRANCE
FEATURES: Promesh® SURG LI is a light-weight mesh design with polypropylene monofilaments large pores to ensure fibrosis and tissue integration. The light-weight structure aligned with a reduced quantity of implanted material will influence the inflammatory reaction and patient post-operative pain. Promesh® SURG LI is cutable and designed for ...
Manufactured by:Crossroads Extremity Systems based inMemphis, TENNESSEE (USA)
There are potential risks associated with the use of these devices, some of which include: allergic reaction to the implant material, fracture of the implant, soft-tissue complication (e.g., infection at the implant site, prolonged healing), and revision surgery. Refer to IFU for all contraindications, warnings, and risks. ...
Manufactured by:Biorez Inc. based inNew Haven, CONNECTICUT (USA)
The BioBrace implant is an innovative bioinductive scaffold that is intended to reinforce soft tissue where weakness exists, and promote soft tissue healing. The BioBrace is cleared by the FDA in K203267. Unlike traditional implant materials that are either synthetic or biologic, BioBrace is a biocomposite of both. ...
Manufactured by:United Orthopedic Corporation (UOC) USA Inc. based inIrvine, CALIFORNIA (USA)
The UDM (United Dual Mobility) acetabular system is designed to help prevent dislocation by providing increased jump distance and range of motion. The UDM acetabular system consists of a Cobalt Chrome (CoCr) acetabular cup and a mobile polyethylene liner that rotates within the cup and captures the femoral head. The inner surface of the cup is polished to allow optimal articulation between the ...
Manufactured by:Neuspera Medical, Inc. based inSan Jose, CALIFORNIA (USA)
The Neuspera micro-implant has a modular construction, enabling adaptation for a variety of neural interfaces. The device has an array of programmable stimulation parameters, including frequency, pulse width, and amplitude (voltage) for current control, allowing for maximum flexibility in addressing common neurostimulation applications. Hermetically sealed and made of standard ...
Manufactured by:Ophtec BV based inGroningen, NETHERLANDS
Artisan Aphakia is the #1 backup lens in complicated cataract cases. Based on the long term experience of iris fixation, the ARTISAN Aphakia IOL is a predictable, safe, high precision implant. ...
Manufactured by:Wenzel Spine, Inc. based inAustin, TEXAS (USA)
A Small Device with Big Potential for your Practice. VariLift®-C is the only expandable interbody fusion device that can be used at 1 cervical level, unilaterally or bilaterally, with or without supplemental fixation, such as plates and ...
Manufactured by:Maruho Medical, Inc. based inMarietta, GEORGIA (US) (USA)
These anchors are cannulated with an integrated suture eyelet. The device is available in varying profile diameters & materials. Each implant is manufactured of all PEEK or a Titanium Eyelet & PEEK body combination. Reusable punches/taps as well as disposable punches for instrumentation are available. ...
Manufactured by:Zavation based inFlowood, MISSISSIPPI (USA)
Internal body has a porous structure, external edges have a solid, roughened surface to engage with the vertebral body end plates, Large graft window through the device, Porous material facilitates osseointegration, Greater contact area at the implant-bone interface*, Material: Titanium per ASTM ...
Manufactured by:Inion Oy based inTampere, FINLAND
The fully threaded Inion FixOn™ Biocomposite Suture Anchor is made of state-of-the-art biocomposite material containing PLGA and osteoconductive β-TCP. The products are provided sterile and preloaded on a practical, single-use driver with two or three nonabsorbable sutures. Inion FixOn™ Biocomposite Suture Anchors provide excellent performance over the healing period in rotator ...
Manufactured by:Hyalex Orthopaedics, Inc. based inLexington, MASSACHUSETTS (USA)
Biomimetic Materials Platform: HYALEX® Cartilage is designed to mimic the form and function of native hyaline cartilage. It has the potential to be applied to products in any articulating joint in the body as well as applications beyond the ...
Manufactured by:Konan Medical USA, Inc. based inIrvine, CALIFORNIA (USA)
Designed to improve patient outcomes, CellChek D+ redefined specular microscopy in Eyebanking. It was quickly and widely adopted by many of the world’s finest Eye ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
Heoliguide is a thin sheet mode of high purity Type-I collagen derived from selected animal tissues and purified using a patented American technology- The additional charge modifications and slight calcification of collagen helps for proper guided bone (issue regeneration. Heoliguide is unique ond different from other native collagen membranes that are known to facilitate fibrogenesis ovec ...
by:OSSIO Inc based inWoburn, MASSACHUSETTS (USA)
Bio-Integrative OSSIOfiber® Hammertoe Fixation System. Innovation and Convenience Delivered In One Package. The combination of the implant plus disposable instrumentation provides easy insertion and secure fixation for proximal interphalangeal (PIP) fusion procedures while utilizing existing surgical ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
The Duo Ti system represents the latest advancements in lateral interbody fusion technology. The system minimizes the amount and duration of retraction on the neural structures and surrounding soft tissues, both of which have been linked to neurologic deficits following lateral interbody fusion surgery.1, ...