Show results for
Refine by
Medical Sterilization System Equipment Supplied In Europe
46 equipment items found
Manufactured by:Molteni Therapeutics s.r.l based inScandicci (FI), ITALY
VULNOFAST® plus is an improved formulation of VULNOFAST® gel 0.3%. VULNOFAST® plus is a sterile medical device (CE0373) topically administered recommended as an adjuvant for the local treatment of skin lesions and ulcers through Photodynamic Therapy. The device is used in combination with a red light source VULNOLIGHT® (led source at 630 ...
Manufactured by:Ahlstrom based inEspoo, FINLAND
Ahlstrom Sterile Barrier Systems (SBS) for sequential wrapping are designed to protect a wide variety of medical devices for optimum patient safety. Ahlstrom’s sterile barrier systems are a trusted and integral part of hospital CSSD. Focused on helping our customers stay ahead, Ahlstrom now offers a ...
Manufactured by:Molteni Therapeutics s.r.l based inScandicci (FI), ITALY
VULNOFAST® gel 0.3% is a sterile medical devices (CE0373) topically administered recommended as an adjuvant for the local treatment of skin lesions and ulcers through Photodynamic Therapy. The device is used in combination with a LED red light source at 630 ...
Manufactured by:Molteni Therapeutics s.r.l based inScandicci (FI), ITALY
ELKOFAST® gel 0.3% is a non sterile medical device (CE0373) topically administered recommended as an adjuvant for the local treatment of Skin lesions and ulcers through Photodynamic Therapy. The device is used in combination with a LED red light source at 630 ...
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
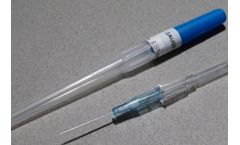
IV cannulas with wing holder / cover, specially designed for one-handed insertion. IV cannulas with wing holder / cover, specially designed for one-handed insertion. The device carries small wings, specially designed for easy gripping and safe clamping to allow secure fixation to ...
Manufactured by:ASANUS Medizintechnik GmbH based inNeuhausen ob Eck, GERMANY
The ASANUS retractor covers STERI COVER have been proving their worth in everyday operating theatres for years - following recertification, they are now available with IIA approval in accordance with Annex II of Directive 93/42/EEC for medical devices. The sterile single use retractor covers from ASANUS offer an easy and quick application due to the secure velcro fastener. Integrated X-ray ...
Manufactured by:Sterimed based inCoulommiers, FRANCE
Ethypel Performance SP 63 gsm is a medical cellulose-based overall coated top web solution designed to provide a controlled and consistent sealing performance for optimized peel ability requirements when combined to films made of a PE seal layer (PA/PE ; PP/PE ; PET/PE, etc.). Ethypel Performance SP 63 gsm is made of a 60 gsm cohesive base sheeting coated with a waterbased 3 gsm adhesive layer ...
Manufactured by:OPIA Technologies based inPARIS, FRANCE
TEARPRIM is a sterile medical device designed to sample the tear film. It enables further analysis of tear components to help diagnose several ocular surface disorders, such as dry eye or allergies. The TEARPRIM device was developed in close collaboration with Quinze-Vingts Hospital and ...
Manufactured by:HAGMED based inRawa Mazowiecka, POLAND
Electrodes are used to recables intracardiac potentials and to temporarily stimulate the heart as a part of an invasive diagnostic electrophysiological examination of the heart (EPS). Electrodes are sterile, disposable medical devices. Electrodes are visible in X-ray imaging and are fully biocompatible. With the use of dedicated HAGMED diagnostic electrode ...
Manufactured by:Ahlstrom based inEspoo, FINLAND
Ahlstrom’s effective bacterial-barrier paper for packaging sterilized medical devices is sealable, peelable and ...
Manufactured by:Beldico based inMarche-en-Famenne, BELGIUM
Transporting organs and tissues for transplantation in complete safety with Beldico sterile bags for organ ...
Manufactured by:OPIA Technologies based inPARIS, FRANCE
EYEPRIM is a sterile medical device designed to perform conjunctival impressions of the living eye. It enables further analysis of cells and biomarkers to help diagnose several ocular surface disorders, such as dry eye, allergies or infections. The EYEPRIM device is based on an original idea, suggested by Christophe Baudouin, MD, PhD, Chairman of Department of Ophthalmology III at Quinze-Vingts ...
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
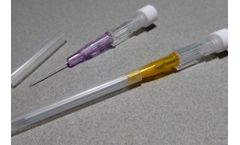
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. Pen-type IV cannulas offer the advantage of potentially fully enclosing the used needle for safe disposal. They are also fitted with a hydrophobic filter. ...
Manufactured by:HAGMED based inRawa Mazowiecka, POLAND
Electrophysiological electrode extension cables are used to connect electrophysiological electrodes (ablation electrodes, diagnostic electrodes for cardiac electrophysiology and electrodes for temporary cardiac pacing) to electrophysiological medical devices, to transfer radio frequency current from medical electrophysiological devices (ablators) to the heart, or to transfer charges/electrical ...
Manufactured by:HAGMED based inRawa Mazowiecka, POLAND
Electrodes are used to recables intracardiac potentials, map the electrical conduction of individual heart cavities, temporarily stimulate the mapped area of the heart, and deliver “RF” current to the myocardium tissue, i.e., ablation of the arrhythmia substrate of the heart. Electrodes are sterile, disposable medical devices. Electrodes are visible ...
Manufactured by:Prodimed based inNeuilly en Thelle, FRANCE
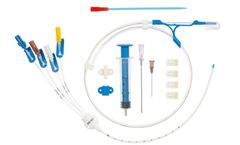
Central venous catheters for introduction by the Seldinger technique. Class III sterile medical ...
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. These IV cannulas are fitted with an injection port that allows the supplementation of a drug into the cannula itself by a syringe without a needle, and prevents ...
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. Also called « one-way » IV cannulas, these cannulas may be supplied, in their sophiticated version, with hydrophobic filters to avoid blood spillage ...
Manufactured by:Meridian Medical based inLittlehampton, UNITED KINGDOM
Our medical equipment manufacturing facilities include 9,000 square feet of clean rooms that offer a mix of ISO class 7 and 8 purpose-built production ...
Manufactured by:HAGMED based inRawa Mazowiecka, POLAND
Electrodes are used for temporary transvenous cardiac stimulation (up to 2 weeks), mainly for resuscitation of patients with ventricular arrest. They can be used in existent or impending bradycardia caused by disease of the conduction system and acute myocardial infarction. Indications for use may include procedures in which a clinically significant decrease in heart rate may occur, as well as ...