- Home
- Equipment
- usa california
- preclinical data from cytotil15 program
Show results for
Refine by
Preclinical Data From Cytotil15 Program Equipment Supplied In Usa California
33 equipment items found
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
CX-188 is our wholly owned PD-1-targeting Probody therapeutic. As with anti-PD-L1 therapies, PD-1 monotherapy and combinations have been associated with significant toxicities. Our preclinical studies have shown that CX-188 has the potential for an improved therapeutic index relative to PD-1 antibodies. The IND for CX-188 was cleared by the FDA. Following a program and portfolio prioritization, ...
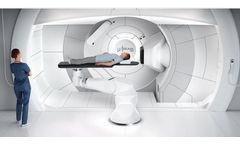
Manufactured by:Varian Medical Systems, Inc. based inPalo Alto, CALIFORNIA (USA)
Next-generation proton therapy with a smaller ...
by:Sorrento Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
Sorrento utilizes a proprietary knock-out knock-in (KOKI) technology to modify normal healthy donor derived T cells to genetically engineer them to express the dimeric antigen receptor into T-cell receptor (TCR) alpha chain constant region (TRAC). In this manner, TRAC is knocked out and antigen is knocked into its locus. The Dimeric Antigen Receptor (DAR) utilizes a Fab instead of the scFv ...
by:Hillhurst Biopharmaceuticals, Inc. based inSan Diego, CALIFORNIA (USA)
Therapeutic medical gases have the potential to address a wide spectrum of diseases, but have been limited by inhaled delivery, which for conscious patients is inaccurate in dosing, potentially unsafe, and highly intrusive. Hillhurst’s GLASS technology platform is designed to address the challenges of inhaled gases by enabling liquid delivery of therapeutic gases to ensure accurate dosing ...
Manufactured by:CytomX Therapeutics, Inc. based inSouth San Francisco, CALIFORNIA (USA)
CytomX is developing CX-2009, a Probody drug conjugate (PDC) conjugated with DM4, a highly potent cytotoxic drug, targeting CD166, also known as ALCAM. CD166 is a molecule widely and highly expressed on solid tumor cells and previously considered “undruggable” given its expression on normal tissues. In preclinical studies, Probody drug conjugates targeting CD166, have led to ...
Manufactured by:Eclipse Regenesis based inMenlo Park, CALIFORNIA (USA)
The Eclipse XL1 is an entirely mechanical and repeatable approach designed to grow healthy intestinal tissue 2-3x longer within 2-3 weeks. Early studies have shown that new tissue has begun to function and absorb nutrients equally (or greater) than existing healthy intestinal ...
Manufactured by:Capricor Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
Our core therapeutic technology (CAP-1002) is based on cardiosphere-derived cells, or CDCs, a cardiac-derived cell therapy that was first identified in the academic laboratory of Capricor’s scientific founder, Dr. Eduardo Marbán. Since the initial publication in 2007, CDCs have been the subject of over 100 peer-reviewed scientific publications and have been administered to ...
Manufactured by:Xenco Medical based inSan Diego, CALIFORNIA (USA)
The Sorrento™ Bone Graft Substitute is a robust, resorbable bone void filler to fill voids or gaps of the skeletal system in the extremities and pelvis not intrinsic to the stability of the bony structure. Pre-clinical animal studies have revealed its tremendous performance through early osteoblast activity and osteocyte formation with little fibrotic tissue. Sorrento™ Bone Graft ...
Manufactured by:Precision NanoSystems based inVancouver, BRITISH COLUMBIA (CANADA)
The NanoAssemblr® Blaze allows rapid scaling of nanoparticle formulations for late pre-clinical development. The system can manufacture between 10 mL and 10 L of formulation which allows researchers to use the Blaze for preclinical toxicology testing, and for early Chemistry, Manufacturing and Controls (CMC) ...
Manufactured by:AVITA Medical based inValencia, CALIFORNIA (USA)
Epidermolysis Bullosa (EB) is a group of rare and incurable skin disorders caused by mutations in genes encoding structural proteins resulting in skin fragility and blistering, leading to chronic wounds and, in some sub-types, an increased risk of squamous cell carcinoma or death. Dystrophic EB is estimated to affect 3-8 million people[1], and there is currently no FDA-approved ...
Manufactured by:Immune-Onc Therapeutics, Inc. based inPalo Alto, CALIFORNIA (USA)
Immune-Onc is evaluating IO-106, an antagonist antibody targeting LAIR1, an immune inhibitory receptor. The company’s preclinical research supports the proposed mechanisms of action of IO-106 and its evaluation as a potential treatment for solid ...
by:Cytori Therapeutics Inc. based inSan Diego, CALIFORNIA (USA)
Cytori is developing cell therapies that harness the unique attributes of living cells that are present in an adult human patient’s own adipose (fat) tissue, also known as Adipose-Derived Regenerative Cells ...
Manufactured by:ClearPoint Neuro, Inc. based inIrvine, CALIFORNIA (USA)
The SmartFlow MR Compatible Ventricular Cannula has received 510(k) clearance from the FDA for use in the US for the aspiration of CSF, or injection of the chemotherapy drug Cytarabine into the ventricle. It has also been CE marked for use in Europe for the delivery of approved fluids into the brain or aspiration of CSF. It’s being utilized in approved clinical and preclinical studies for ...
Manufactured by:Fibralign Corporation based inUnion City, CALIFORNIA (USA)
Fibralign is developing a pipeline of compelling novel products which are based on its proprietary Nanoweave® scaffolding platform that can be tailored to address a wide range of high-value applications. Nanoweave technology provides the means to precisely print 3D scaffolding in such a way that mimics human tissue nano structure and directly influence the body’s repair ...
Manufactured by:Galvanize Therapeutics, Inc. based inRedwood City, CALIFORNIA (USA)
The Aliya PEF System is designed for the surgical ablation of soft tissues by delivering high voltage, short duration electrical energy. This targeted electrical energy alters the transmembrane potential, leading to the disruption of cellular homeostasis and inducing non-thermal programmed cell death. Utilizing technologies such as the Aliya Needle and the INUMI™ Flex Needle, the system ...
Manufactured by:Actym Therapeutics based inBerkeley, CALIFORNIA (USA)
Actym Therapeutics, based in Berkeley, CA, is a privately held biotechnology company focused on the discovery and development of novel immunotherapies to treat cancer. The company has developed an attenuated, microbial-based, technology platform called STACT (S. Typhimurium-Attenuated Cancer Therapy). In preclinical studies, STACT specifically enriches in many types of solid tumors and not in ...
Manufactured by:Bruker Corporation based inBillerica, MASSACHUSETTS (USA)
Designed for the emerging market of preclinical and molecular MR imaging: The BioSpec series is designed for the emerging market of preclinical and molecular MR imaging. State-of-the-art MRI CryoProbe technology together with ultra high field USR magnets deliver high spatial resolution in vivo enabling customers to come closer to the molecular and cellular level research they desire. Thanks to ...
Manufactured by:CeloNova BioSciences, Inc. based inCarlsbad, CALIFORNIA (USA)
CeloNova develops and manufactures novel surface coated technologies including its proprietary Polyzene-F nanocoating and COBRA SG Drug-Coated PTCA Balloon technology. This next-generation innovation is transforming clinical practice and promoting positive outcomes in patients around the ...
Manufactured by:Biocardia, Inc. based inSunnyvale, CALIFORNIA (USA)
Leading physicians have described the current Helix Biotherapeutic Delivery System as elegant in its simplicity. BioCardia’s Helix Biotherapeutic Delivery System is the leading percutaneous catheter delivery system for cardiovascular regenerative medicine. It enables local delivery of cell and gene based therapies to treat heart failure, myocardial infarction, ischemia, and cardiac ...
Manufactured by:Galvanize Therapeutics, Inc. based inRedwood City, CALIFORNIA (USA)
The Aliya PEF System is an advanced medical device used primarily for the surgical ablation of soft tissues, delivering high voltage, short-duration electrical energy to target cells. The system employs pulsed electric field technology to disrupt cellular homeostasis, leading to non-thermal programmed cell death while maintaining the integrity of the surrounding extracellular matrix. The system ...