Only show results in California? Ok
Pharmaceutical Software
-
based in USA
The starting point are the national regulations of the United States and the European ...
-
Manufactured by Charles Ischi AGbased in SWITZERLAND
Ph21 Software is designed for comprehensive quality assurance in the pharmaceutical industry, capable of controlling and evaluating various testing devices centrally. It supports up to 32 external pharmaceutical testing devices, including those from Kraemer Elektronik like UTS tablet testing systems and disintegration testers. ...
-
based in SWITZERLAND
This powerful detailed scheduling software was developed specifically for the needs of the process industries (chemicals, pharmaceuticals, cosmetics, food, etc.). It offers the planner a detailed visualization of the schedule as well as the possibility to keep at each moment an overview over all resources needed for production (devices, manpower, raw materials,...). ...
-
Manufactured by Particle Measuring Systems (PMS)based in USA
The top Data Collection and leading Data Management companies combined to create a complete contamination monitoring solution for pharmaceutical manufacturers. Plus, with its unique plug and play ability, portable devices and monitoring, system connection has never been ...
-
by Intelligenbased in USA
SuperPro Designer facilitates modeling, evaluation and optimization of integrated batch and continuous processes in a wide range of industries (Biotech, Pharmaceutical, Specialty Chemical, Food Processing, Consumer Goods, Metallurgical, Materials, Water Purification, Wastewater Treatment, Air Pollution Control, etc.). The combination of manufacturing and environmental ...
-
based in INDIA
Pharmaceutical companies are required to submit a full Product Quality Review (PQR) or APQR in the U.S. and PQR in Europe to government health authorities annually, detailing key aspects of the manufacturing process for each drug they produce. ...
-
Manufactured by Otsuka America Pharmaceutical, Incbased in USA
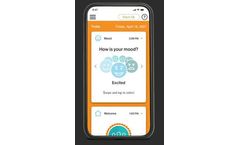
The MYCITE App receives and displays the information from the Bluetooth patch. On the app, you can also record your mood, how well you rested, and the reason, if you didn’t take your pill. You can look at all this information at any time to see your medication routines and ...
-
Manufactured by Otsuka America Pharmaceutical, Incbased in USA
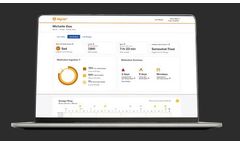
The MYCITE Dashboard is an online portal that lets your healthcare team and your support network see the data you share with them, like when you took your ABILIFY ...
-
based in UNITED KINGDOM
Virtual Tumour™ is our advanced, predictive modelling simulation tool used worldwide by global pharmaceuticals to small biotech companies to support oncology ...
-
by Bayoonet AGbased in GERMANY
For 10 years we have been implementing software projects for the pharmaceutical and medical technology sector in accordance with the applicable regulatory standards IEC 62304, ISO 14971 and IEC 60601-1-6 / IEC ...
-
Manufactured by Vimachembased in USA
The Vimachem Pharma MES Platform offers an advanced Manual Aggregation Module specifically tailored for pharmaceutical applications. This software solution is a crucial component of Vimachem’s open serialization platform, facilitating seamless connectivity with various IT systems and third-party equipment. Designed for manual, low-speed lines, the module supports the manual ...
-
based in GERMANY
Right First Time", "Review by Exception", "Paperless Production", "Electronic Batch Recording", "Faster Time to Market", "FDA Compliance" – these are only some of the benefits of using Werum's out-of-the-box MES software product PAS-X for your pharmaceutical and biopharmaceutical ...
-
by Instem Groupbased in UNITED KINGDOM
NOTOCORD-hem™, developed by NOTOCORD, and now part the Instem solutions portfolio is the leading software platform for the acquisition, display and analysis of physiological signals, with a focus on the areas of Cardiovascular, Respiratory and Nervous System Research. NOTOCORD-hem is used by pharmaceutical companies, contract research laboratories, hospitals and academic ...
-
based in USA
With unmatched ease-of-use and scalability, powerful analytic capabilities, and the fastest processing speeds available for digital pathology, pharmaceutical, healthcare and research organizations worldwide are using HALO for high-throughput, quantitative tissue analysis in oncology, neuroscience, metabolism, toxicology and more. ...
-
by TriNetX, LLCbased in USA
Trials that include patients in Japan have an advantage when it comes to regulatory approval in that country, where pharmaceutical sales reached 94B USD in 2020. But approval requires promising results from a well-executed study. Start the process the right way by understanding Japan's patient population, designing a feasible study, and selecting the right trial sites. ...
-
based in CANADA
In clinical studies, assessing and managing risks, as well as establishing areas of uncertainty that contribute to risk, are critical to a company’s decision making processes. Many business units within pharmaceutical, biotechnology and clinical research organizations must comply with federal regulations concerning their processes and computerized systems. One of the most ...
-
by Certarabased in USA
The Simcyp Simulator is the pharmaceutical industry’s most sophisticated physiologically based pharmacokinetics (PBPK) platform for determining first-in-human dosing, optimizing clinical study design, evaluating new drug formulations, setting the dose in untested populations, performing virtual bioequivalence analyses, and predicting drug-drug interactions (DDIs). ...
-
Manufactured by Syncro Medicalbased in USA
The global life sciences/diagnostic instrumentation market is estimated to be $55 Billion today, and continues to grow at a rapid clip, according to Business Wire. The growth of this market is driven largely by increasing spending on pharmaceutical R&D for a growing number of research studies related to drug discovery and development. The increasing demand for diagnostic ...
-
based in USA
The ASAPprime software is the only commercially-available software that enables pharmaceutical scientists to quickly and accurately determine drug substance and drug product shelf-life based on the Accelerated Stability Assessment Program (ASAP). This state-of-the-art, statistical software allows prediction of small-molecule drug product use periods, shelf-lives and drug ...
-
Distributed by Aseptconn AGbased in SWITZERLAND
AseptSoft is the leading provider of process design software for engineering companies and end-users in the (bio)pharmaceutical industry. In an era of digital transformation, we empower our clients to stay ahead of the curve by transforming manual process design into an interactive, digital platform. Our comprehensive solution integrates seamlessly as a plugin with existing ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you